Zinc-air batteries consist of an anode of zinc and a cathode of oxygen from the air. The batteries are cheaper to produce than lithium-ion batteries because there is plenty of zinc. In addition, zinc-air batteries have the potential to store more energy – theoretically five times as much as lithium-ion batteries – while zinc air is safer and more environmentally friendly.
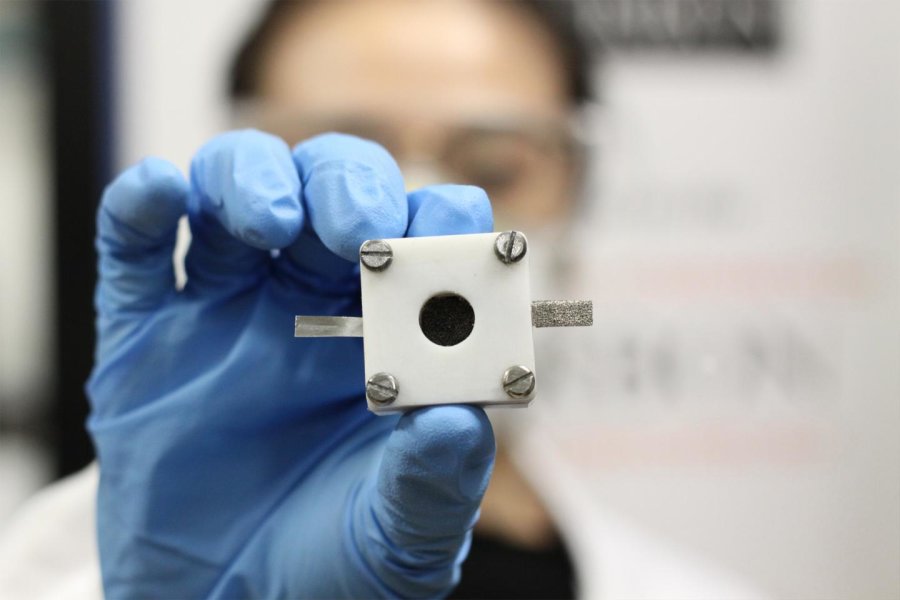
Credit: The University of Sydney
Zinc-air batteries are used in a few electronic devices, but since it has been difficult to produce rechargeable zinc air batteries, they have not yet received a broader impact.
Charging is difficult because the battery does not have electrocatalysts that can generate oxygen during charging and reduce the amount of oxygen upon discharge.
Now, researchers at the University of Sydney and Nanyang University of Technology have presented a three-step approach to overcome this problem. The method was recently featured in the journal
Advanced Materials.
“Up until now, rechargeable zinc-air batteries have been made with expensive precious metal catalysts, such as platinum and iridium oxide. In contrast, our method produces a family of new high-performance and low-cost catalysts,”
– Lead author Professor Yuan Chen, from the University of Sydney in a press release.
The new catalysts are made of metal oxides from common substances such as iron, cobalt, and nickel. These new catalysts are produced through the simultaneous control of the: 1) composition, 2) size and 3) crystallinity of metal oxides of earth-abundant elements such as iron, cobalt, and nickel. They can then be applied to build rechargeable zinc-air batteries.
“We are solving fundamental technological challenges to realise more sustainable metal-air batteries for our society,”
– Professor Chen Chen.
Testing of batteries with the new catalysts has shown good results in terms of rechargeability, according to the researchers. The batteries dropped less than ten percent of their capacity after 60 charging cycles.
Reference:
Li Wei, H. Enis Karahan, Shengli Zhai et al. Amorphous Bimetallic Oxide–Graphene Hybrids as Bifunctional Oxygen Electrocatalysts for Rechargeable Zn–Air Batteries DOI: 10.1002/adma.201701410









![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55136/b1b0b614-5b72-486c-901d-ff244549d67a-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55124/79bc18fa-f616-4951-856f-cc724ad5d497-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55099/2638a982-b4de-4913-8a1c-1479df352bf3-350x260.webp)