A new method to easily desalinate water with high efficiency has been developed by researchers at The University of Saarland. It could potentially make seawater a viable source of freshwater to drink.
In a world where drinkable water is increasingly an aspect correlated with conflict and disparity. As global warming and population growth are forcing more people to share a dwindling amount of freshwater. Therefore, any breakthrough technology that makes the abundant amounts of seawater on our blue planet a viable source of drinkable water would truly be revolutionizing.
The trick though is to find a method that is efficient, relatively cheap and simple. Most known forms of declination are neither. Methods such as thermal declination, vacuum declination or reversed osmosis.
A technology that would fulfill these three criteria’s would essentially make seawater drinkable water in those areas of the world where it is needed the most, which often implies poor and developing countries.
Now, researchers at the German University of Saarland has been developing a new method that one of the researchers describe as almost too good to be true.
The technology called “capacitive deionization” does not only desalinate salt water into freshwater, it would also potentially serve as an energy storage technology for variable energy production sources, such as solar power and wind power.
The German researchers are not alone in researching this particular technology. It is currently being researched across the world but is still in its infancy stages.
The concept of electrochemical demineralization of water was first reported in the 1960s by Blair and Murphy. In 1970 Johnson et al.introduced a theory for the process called “potential modulated ion sorption”. In 1996, Farmer et al. also introduced the term “capacitive deionization” and used the now commonly abbreviation “CDI” for the first time. In 2004, Membrane Capacitive Deionization was introduced in a patent of Marc Andelman.
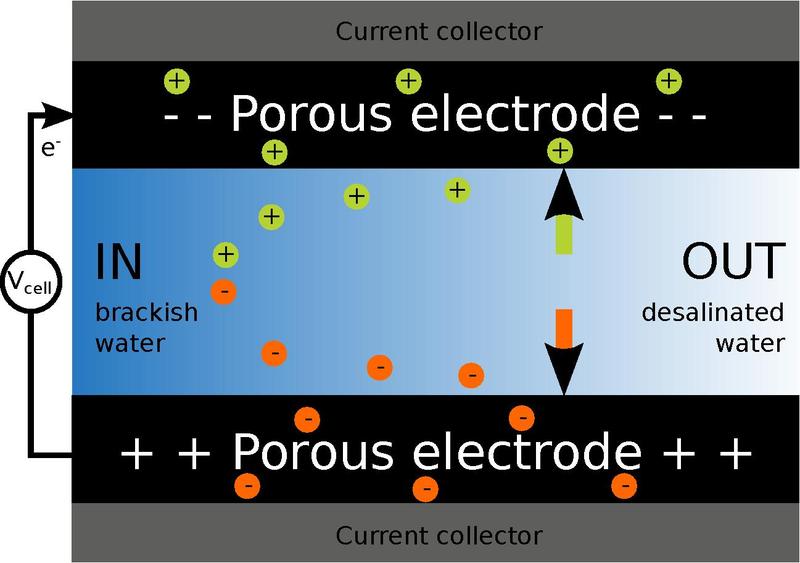
The basic principle is to deionize water by applying an electrical potential difference over two porous carbon electrodes. The positively charged electrode attracts negatively charged ions from the water and the negatively charged electrode attracts positively charged particles from the water.
The charged particles are stored in the porous carbon, and the end result of the process is fresh water. With an efficiency of 80 percent and with a potential of combining this simple physical principle with membranes between the water and the carbon electrodes that would potentially increase the efficiency to 95 percent according to the researchers.
The electrodes can be cleared of ions when “full”, resulting in a highly concentrated salt solution.
But the method is not limited to desalination, as ultracapacitors, the technology could eventually be a positive input into the power industry in general, the solar electric power industry and the wind power industry specifically, providing grid smoothing, making these energy sources more attractive and providing a new reliable way of energy storage.
______________
Carbon flow electrodes for continuous operation of capacitive deionization and capacitive mixing energy generation
Ultracapacitor Storage Can Help Build A Reliable Solar Grid
Charge barrier flow-through capacitor
____________________________











![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55136/b1b0b614-5b72-486c-901d-ff244549d67a-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55124/79bc18fa-f616-4951-856f-cc724ad5d497-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55099/2638a982-b4de-4913-8a1c-1479df352bf3-350x260.webp)