Researchers in Singapore have made a breakthrough in hydrogen fuel cell research. According to the team – this will make fuel cells both cheaper and more stable.
Fuel cells are a technology that converts chemical energy from a fuel (hydrogen) into electricity through a chemical reaction with oxygen or another oxidizing agent. The technology is very attractive from an environmental perspective, with fuel cell powered cars generating only one byproduct – water.
Explosive and Expensive
There are some drawbacks with the technology, however. Hydrogen itself is somewhat dangerous, being very reactive and thereby explosive. Then there is a need of using precious metals in the making of the fuel cells, eg. platinum. These act as catalysts during the reaction and generation of electricity. And are also needed to sustain the reaction in an acidic environment.
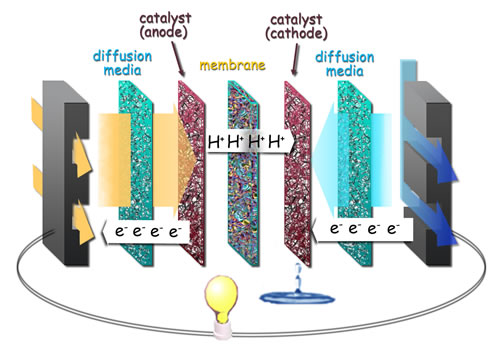
These drawbacks are what the researchers at IBN (Insitute of BioEngineering and Nanotechnology) in Singapore have managed to mitigate. They have developed a cheaper alternative to platinum. A catalyst that consists of gold and copper instead, and then covered with only a thin layer of platinum.
The result is a much more effective catalyst process. Since the nanocomposite material can produce at least 0.571 amperes of electric current per milligram of platinum, compared to 0.109 amperes per milligram of platinum for commercial platinum catalysts.
The study Stabilization and Compressive Strain Effect of AuCu Core on Pt Shell for Oxygen Reduction Reaction has been published in the journal Energy & Environmental Science
The storage issue was addressed last year when another research breakthrough by a team of researchers at USC (University of Southern California) created a possible solution in storing hydrogen in a much safer chemical form. To then be released as pure hydrogen from this innocuous chemical material, a combination of nitrogen, boron and ammonia borane.
This team then developed a catalyst system that releases enough hydrogen to make the chemical material work as a fuel source. This system is also air-stable and reusable, unlike other systems incorporating boron and metal hybrids. The results were published in the Journal of the American Chemical Society.
Brian L. Conley, Denver Guess, Travis J. Williams. A Robust, Air-Stable, Reusable Ruthenium Catalyst for Dehydrogenation of Ammonia Borane. Journal of the American Chemical Society, 2011; 110818113439060 DOI
____________________________











![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55136/b1b0b614-5b72-486c-901d-ff244549d67a-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55124/79bc18fa-f616-4951-856f-cc724ad5d497-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55099/2638a982-b4de-4913-8a1c-1479df352bf3-350x260.webp)