What is the carbon 14 method?
It’s a method often used by scientists to determine the age of archaeological objects, remains from humans, animals, and plants.
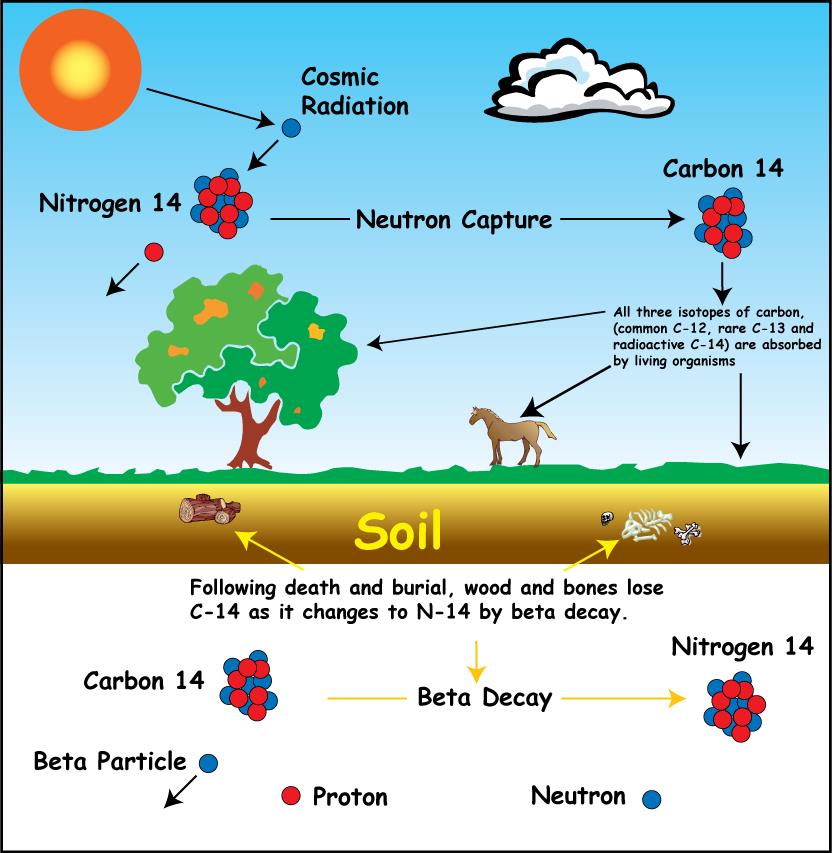
How does it work?
A stable variant of carbon has been denoted 12C, C-12 or carbon-12. But besides this molecule of carbon, there is also another version called carbon-14, containing 6 protons and 8 neutrons.
C-14 is present in the air and is therefore absorbed by all living organisms, plants and animals alike. The proportion of C-12 to C-14 is the same in the atmosphere as long as the organism lives.
But when the organism dies, it ceases to absorb carbon dioxide and since C-14 is unstable it starts to dissolve. The half-life of C-14 is approximately 5,700 years. So after 5,700 years, the amount of C-14 is half.
Therefore, by measuring the ratio of C-12 to C-14 in a sample from the organism, it is possible to determine how many years have passed since the organism died.
Estimating a more exact age is harder the older the specimen is, and uncertainty is therefore expressed by using a range of age statement.
The method was invented in 1948 by Willard Libby of the University of California. He received a Nobel Prize in Chemistry for his discovery in 1960.








![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55136/b1b0b614-5b72-486c-901d-ff244549d67a-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55124/79bc18fa-f616-4951-856f-cc724ad5d497-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55099/2638a982-b4de-4913-8a1c-1479df352bf3-350x260.webp)