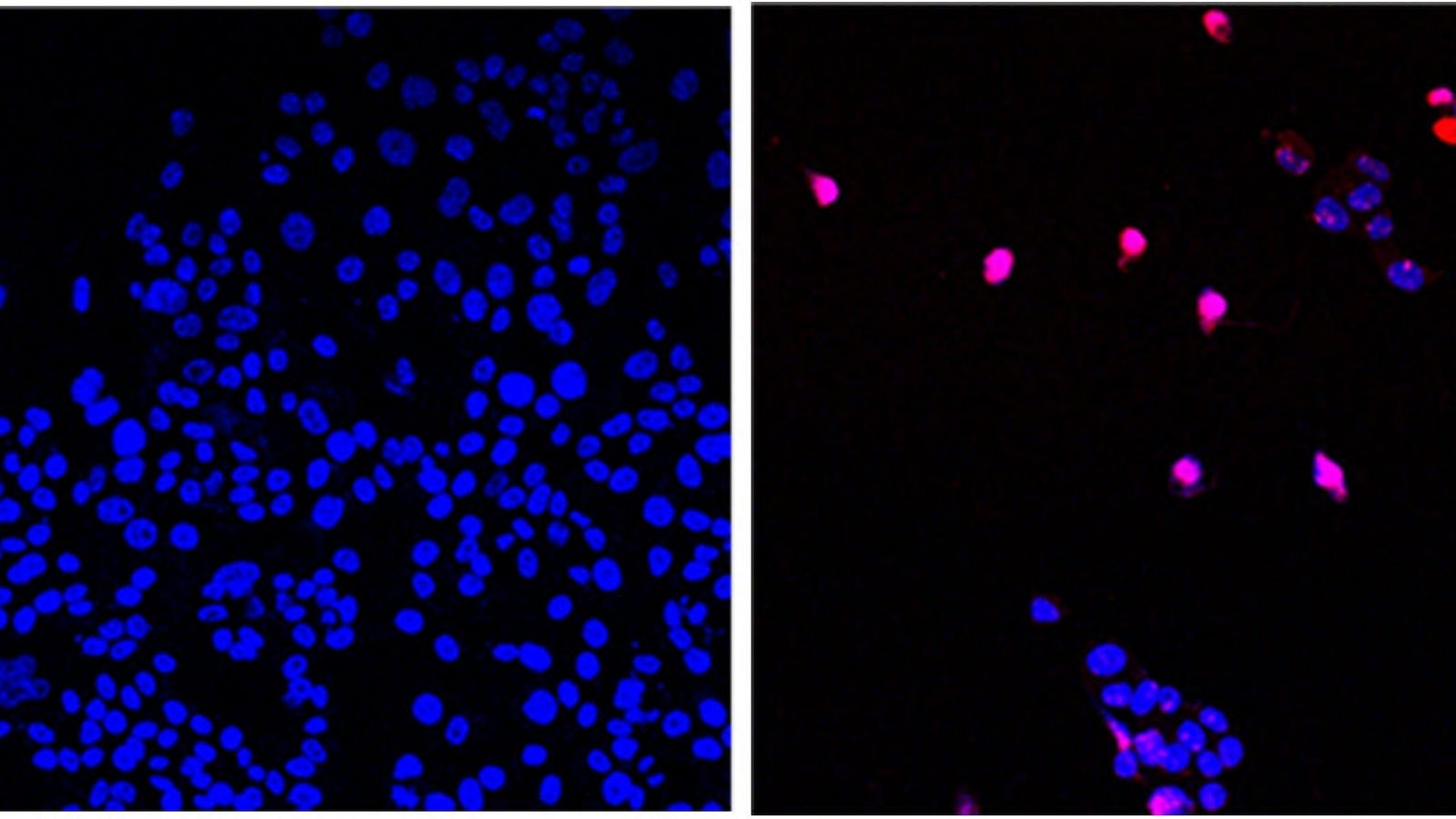
In an astonishing development that holds the promise of changing the landscape of cancer treatment, scientists have made a significant breakthrough in the quest for a potential cure. Researchers have identified a novel approach that targets the protein ‘proliferating cell nuclear antigen’ (PCNA), offering new hope in the fight against cancer and marking a critical milestone in oncology research.
Understanding the Role of PCNA in Cancer
Proliferating cell nuclear antigen (PCNA) is a crucial protein involved in DNA replication and repair, making it essential for cell division and growth. While PCNA is vital for normal cell function, it is often overexpressed in cancer cells. This overexpression fuels the uncontrolled growth and division of cancer cells, contributing to tumor progression and metastasis.
In recent years, scientists have been exploring ways to harness this specific characteristic of cancer cells to develop targeted therapies that attack PCNA, potentially leading to the development of a potent cure for various types of cancer.
The Breakthrough: A Promising Targeted Therapy
The recent breakthrough centers around a new class of drugs known as PCNA inhibitors. Researchers have successfully identified and tested small molecules capable of binding to PCNA, inhibiting its function and disrupting cancer cell growth. By directly targeting PCNA, these inhibitors represent a highly focused and precise treatment strategy.
Initial preclinical trials have shown remarkable efficacy and selectivity of PCNA inhibitors, as they specifically target cancer cells while sparing healthy cells. This specificity is a vital factor in reducing adverse side effects often associated with conventional cancer treatments like chemotherapy and radiation therapy.
Promising Results and Ongoing Research
Researchers have tested PCNA inhibitors in a variety of cancer types, including breast, lung, colon, and pancreatic cancer. In animal models, the inhibitors have displayed an unprecedented ability to halt tumor growth and, in some cases, even lead to complete regression of tumors.
However, it is essential to approach these findings with cautious optimism, as more extensive and rigorous clinical trials involving human subjects are still needed. The transition from preclinical studies to human trials will be a critical step in validating the efficacy and safety of PCNA inhibitors. If the results continue to be promising, these inhibitors could potentially become an integral part of the oncologist’s toolkit.
Future Implications and Challenges
The potential cure for cancer targeting PCNA offers hope for patients worldwide, promising more effective and less invasive treatments with fewer side effects. If approved and commercialized, this breakthrough could significantly improve the quality of life for cancer patients and increase their chances of long-term remission.
However, it is crucial to acknowledge that scientific breakthroughs often come with challenges and hurdles. The road to approval and widespread adoption of PCNA inhibitors may involve overcoming regulatory requirements, scaling up production, and addressing potential drug resistance issues.
Moreover, cancer is a complex and multifaceted disease, and even if PCNA inhibitors prove to be successful, they may not be a panacea. Researchers will continue exploring combination therapies and personalized treatment approaches to tackle the heterogeneity of cancer and improve patient outcomes further.
In Conclusion
The recent breakthrough concerning a potential cure for cancer, targeting the protein PCNA, has ignited excitement and hope within the medical and scientific communities. The development of PCNA inhibitors represents a groundbreaking step towards more targeted and effective cancer treatments.
While more research is needed to translate these findings into transformative treatments for patients, the discovery of PCNA inhibitors holds immense promise. It serves as a reminder of the tireless efforts and dedication of scientists in their pursuit of understanding cancer and developing life-saving therapies.
As clinical trials progress and more data becomes available, we remain cautiously optimistic that the potential cure for cancer may finally be within reach, offering renewed hope to millions of patients and their families affected by this devastating disease.
The Phase 1 clinical trial testing the safety of a potentially cancer-stopping therapeutic developed by City of Hope in people with reoccurring solid tumors is expected to continue for the next two years.







![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55136/b1b0b614-5b72-486c-901d-ff244549d67a-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55124/79bc18fa-f616-4951-856f-cc724ad5d497-350x260.webp)
![OpenAI. (2025). ChatGPT [Large language model]. https://chatgpt.com](https://www.illustratedcuriosity.com/files/media/55099/2638a982-b4de-4913-8a1c-1479df352bf3-350x260.webp)